


100 testů
Venor®GeM qEP is used for direct detection of Mollicutes (Mycoplasma, Acholeplasma, Spiroplasma) contamination in cell cultures and cell media components.
37 770 Kč bez DPH
Venor®GeM qEP utilizes quantitative, real-time PCR (qPCR). The kit can be performed with any type of real-time PCR cycler able to detect the fluorescence dyes FAM™ and HEX™. The protocol provided is preferred for fast and reliable screening of cell culture supernatants most applicable in research and development. The detection procedure can be performed within 3 hours.
Mycoplasmas are specifically detected by amplifying a highly conserved rRNA operon, or more specifically, the 16S rRNA coding region in the mycoplasma genome. The mycoplasma-specific amplification is detected at 520 nm (FAM™ channel).The kit includes primer and FAM™ labeled probes which allow the specific detection of all Mollicutes species so far described as contaminants of cell cultures and media components. Eukaryotic DNA is not amplified by this primer/probe system.
False negative results due to PCR inhibitors or improper DNA extraction are detected by the internal amplification control which can be added to the PCR master mix. The amplification of the internal amplification control is detected at 610 nm (HEX™ channel).
TaqMan® based qPCR Assay with FAM™ and HEX™ labeled probes
Venor®GeM qEP is used for direct detection of Mollicutes (Mycoplasma, Acholeplasma, Spiroplasma) contamination in cell cultures and cell media components.
lyophilized primer/nucleotide/probe/polymerase/mix in aliquots of 25 tests
rehydration buffer
lyophilized internal amplification control
lyophilized positive control
PCR grade water
cycler based, real-time PCR
PCR reaction tubes
Optional for process validation and EP 2.6.7 compliant testing:
Internal Control DNA extra (4 vials for 300 µl each of internal amplification control; Cat. No. 11-9905)
10CFU™ Sensitivity Standards available for all EP 2.6.7 listed mycoplasma species
qPCR cycler with FAM™ and HEX™ filter
variable microliter pipettes
benchtop centrifuge for 1.5 ml reaction tubes
Components can be stored at +2 to +8 °C for at least 12 months. After rehydration the reagents must be stored at -18 °C.
Yes.
Please note that validation data are provided for information purpose only. EP 2.6.7 clearly states “Where commercial kits are used …, documented validation points already covered by the kit manufacturer can replace validation by the user. Nevertheless, the performance of the kit with respect to its intended use has to be demonstrated by the user (e.g. detection limit, robustness, cross-detection of other classes of bacteria.”. Please feel free to contact us if you need further assistance.
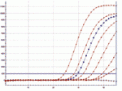
Fig. Amplified dilution series of Mycoplasma fermentans, performed on a
Corbett RotorGene®6000.